02/11/22 - Intro to Hypoxia and Shear Stressing
- Cell Signaling
- Force generated by blood flow in the endothelium (thin membrane lining inside of blood vessels)
- Cell signaling – one cell releases a signaling molecule that is received by a target cell. Can lead to cascade in target cell
- The lack of shear stress leads to the opening of gap between the cells
- The stress fibers, when they contract, the cells contract. The cells contract and you have the opening of the gap and fluid can pass
- Shear stress: force when particles hit the walls of endothelial cells
- Shear stress is important to regulate inflammatory processes
- influences vascular inflammation
- Cells/tissues lack oxygen
- Can lead to tachycardia, seizures, or death
- Shear stress is beneficial, but too much or too little is bad (Ex: high blood pressure = too much; too little = bad during lung transplant –> failure)
- Examined cell signaling pathways
- NF-kB
- HIF
- Reviewed truth tables + network symbols
- A | B –> true
- A or B has to be true in order for product to be true
- A & B –> true
- both A and B have to be true
- A –> B = A promotes B
- A –| B = A inhibits B
- A | B –> true
Homework: Read a research paper on shear stressing
02/18/22 - Understanding Shear Stress
Purpose of reading paper (mentioned in last week’s entry): understanding the mechanosignaling cascade helps design strategies to reduce injury with lung transplant/can increase viability of lungs
Key terms:
Mechanotransduction – signaling cascade caused by the stopping blood flow that shows a physical event sensed by the pulmonary endothelium, a thin membrane that lines the inside of the heart and blood vessels. AKA: converting a physical signal to a chemical one
Mechanosome – stop of flow is sensed by this and has receptors and when activated results in cell membrane depolarization → NADPH oxidase (NOX2) → reactive oxygen species (ROS).
Findings:
Study looks to understanding flow sensing and signaling to derive lessons for application in clinical situations. Lung ischemia (stop of flow) during ventilation activates a mechanosignaling cascade that leads to the production of ROS. This cascade is initiated by a mechanosome, a mechanosensing complex, which is transmitted by the endothelial cell membrane depolarization and that results in NOX2 generation. This is important to preserve donor lungs since they experience a lack of oxygen, or hypoxic state
Meeting Notes:
Reviewed the code to start building our own network. Downloaded R and R studio. Learned how to create graphs and truth tables (or boolean statements) in our code.
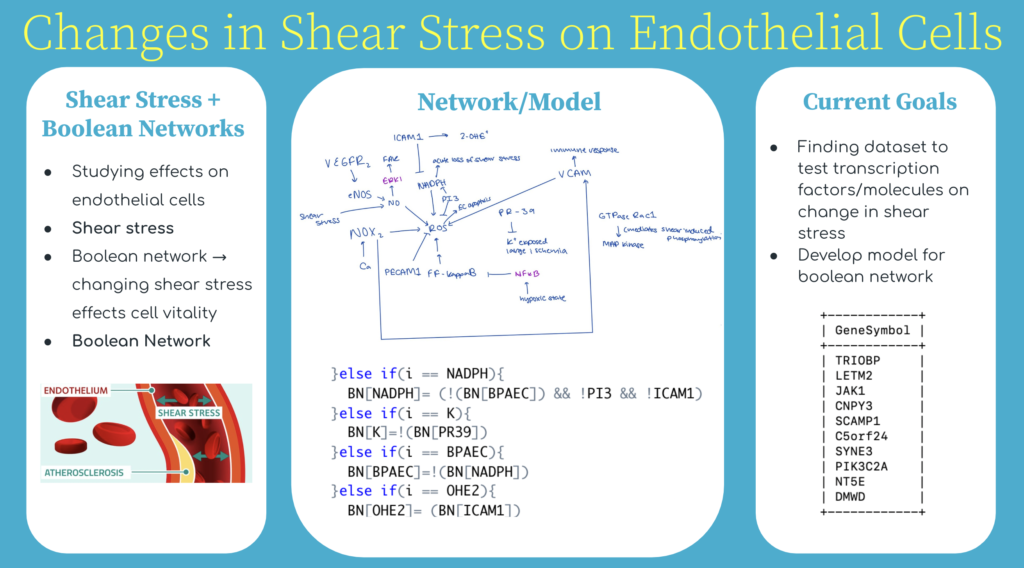
03/04/22 - Writing an Introduction/Editing Code
Continued to read more research papers to understand shear stress and learn how to add to the networking model. Learned how to edit my code and model to make it more accurate. Wrote an introduction on recent findings. Built general model of what change in shear stress could look like as signaling cascade
Part of Introduction:
“Pulmonary endothelial cells (ECs) line the interior of blood vessels and facilitate pulmonary circulation of nutrients and fluid transfers [1]. Endothelial cell vitality is directly affected by shear stress, a physical force or pressure against the cell walls, through the transduction of a series of intracellular signals (a signaling cascade), often noted as mechanotransduction. Shear stress plays a vital role in regulating pressure within the cell wall and has been seen to contribute to the ECs health [3]. A change in shear stress, due to the environment being in a hypoxic or ischemic state, has been seen to have effects on the endothelium and consequently, the endothelial cells. This study aims to understand the chemical pathway that is affected by shear stress, in relation to endothelial cells, to ultimately create a mathematical model and network to represent the effects of manipulating shear stress-related mechanisms on the endothelial cells.
Changes in shear stress initiate cascades that are received by the endothelium via mechanasomes and molecules such as PECAM and VEGFR2; this “message” results in NADPH activation, intracellular Ca2+, eNOS activation, and reactive oxygen species (ROS) like hydrogen peroxide production. When there is too much shear stress, in other words, too much production of ROS, there is a chance of the ECs becoming damaged and negative implications for cellular functions [6]. Excess ROS production can lead to autoimmune diseases, oxidative injury, or inflammatory cells. By that same token, the stop of shear stress, a hypoxic state in which there is no blood flow, often the case in transplants, leads to a reduction in nitric oxide, NO, and eNOS expression, both crucial to protecting and regulating the ECS…”
Sources Referenced:
Johns Hopkins. “Long Term Complications.” Johns Hopkins University. https://www.hopkinsmedicine.org/transplant/programs/lung/_docs/4_Lung_Guide_longterm_complications.pdf.
Chatterjee, Shampa, and Gary F. Niema et al. “Shear stress-related mechanosignaling with lung ischemia: lessons from basic research can inform lung transplantation.” Sep. 19, 2014. Am J Physiol Lung Cell Mol Physiol, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4217004/.
Yi-Shuan J Li et al. “Molecular basis of the effects of shear stress on vascular endothelial cells.” Oct. 2005, PubMed, https://pubmed.ncbi.nlm.nih.gov/16084198/#:~:text=Endothelial%20cells%20(ECs)%20are%20subjected,as%20well%20as%20gene%20expression.
Russo T.A. et al. “Altered shear stress on endothelial cells leads to remodeling of extracellular matrix and induction of angiogenesis.” Nov 19, 2020. Plos One. https://journals.plos.org/plsone/article?id=10.1371/journal.pone.0241040#:~:text=Endothelial%20cells%20(ECs)%20are%20subjected,morphology%2C%20physiology%20and%20gene%20expression.
Uzarski Joseph S.et al.“Adaptation of Endothelial Cells to Physiologically-Modeled, Variable Shear Stress.” Feb 14, 2014. PubMed. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3573044/
Homework: expand on model through literature reviews and work on code (using R)
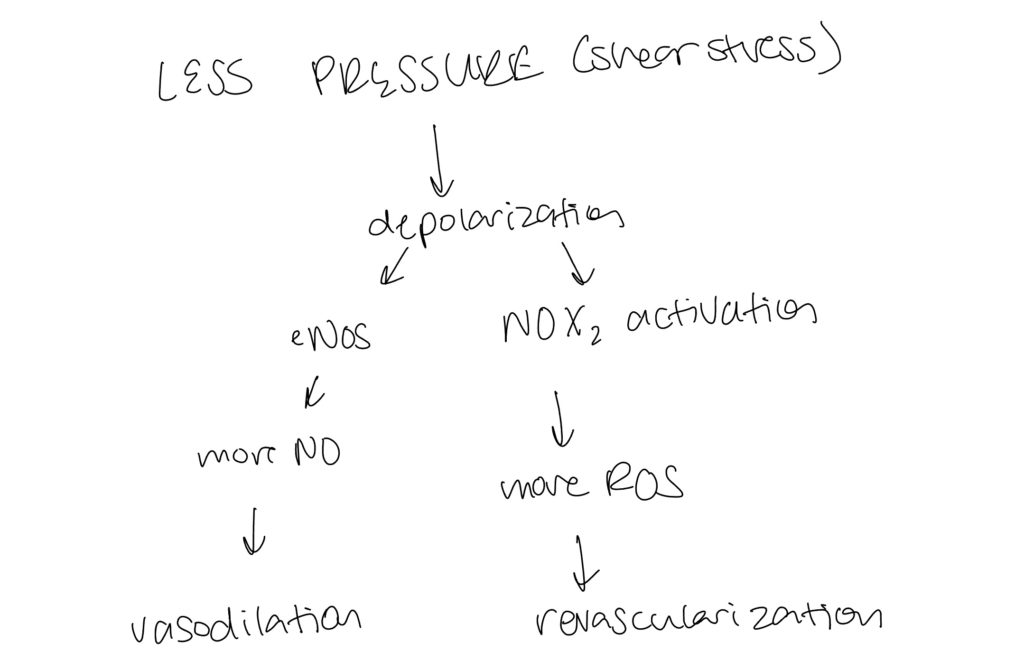
03/11/2022 - Adding to Network
Added to boolean network updated code to incorporate the truth tables of the drawn connections. Read more articles to determine connections, especially from ROS. Recent articles determined a change in shear stress is too broad (an increase in shear leads to different signals compared to low shear stress)– may need to diverge and focus on low shear stress since there is not that much literature out there on this. Interesting connections were COX2, the role of shear stress in relation to cell angiogenesis and cell migration.
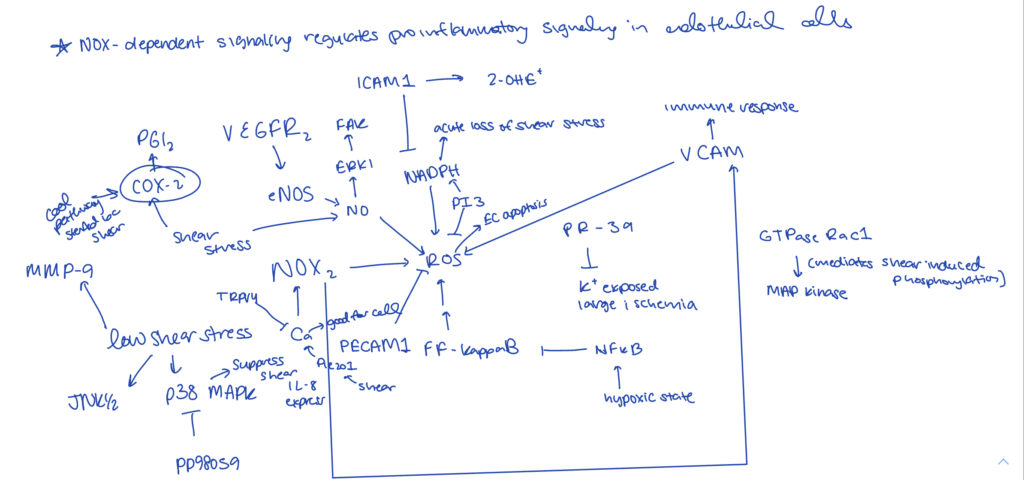
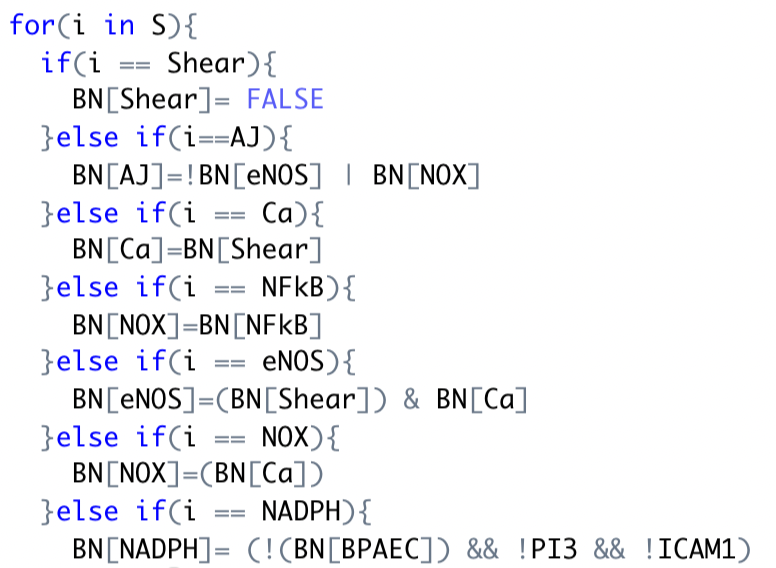
As shown on the left, began to update the connections to boolean statements using R code.
03/18/2022 - Updating Network to Low Shear Stress
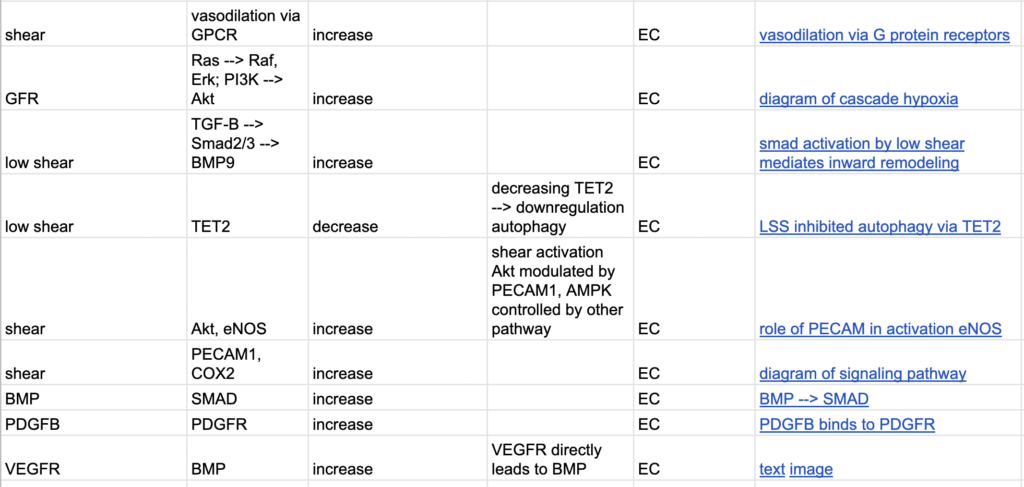
After meeting with Melody and Henrique, determined focusing on low shear stress would be more accurate/useful compared to change in shear stress. Developed new model that related to signaling pathways affected by low shear stress. Worked on annotated bib by creating input/output tables– helped with finding papers that were experiments not just summary papers.
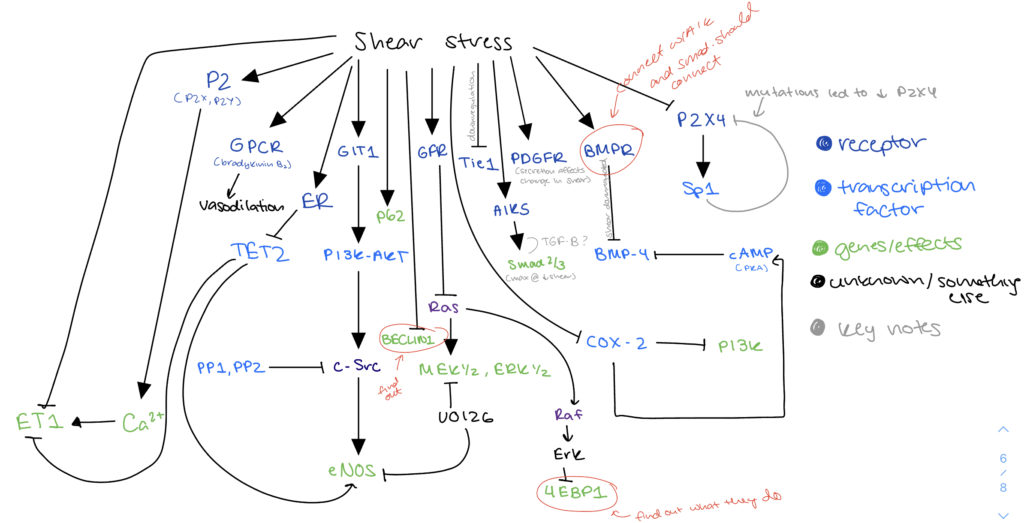
As shown below, began a spreadsheet to document the inputs and outputs that would justify the boolean network.
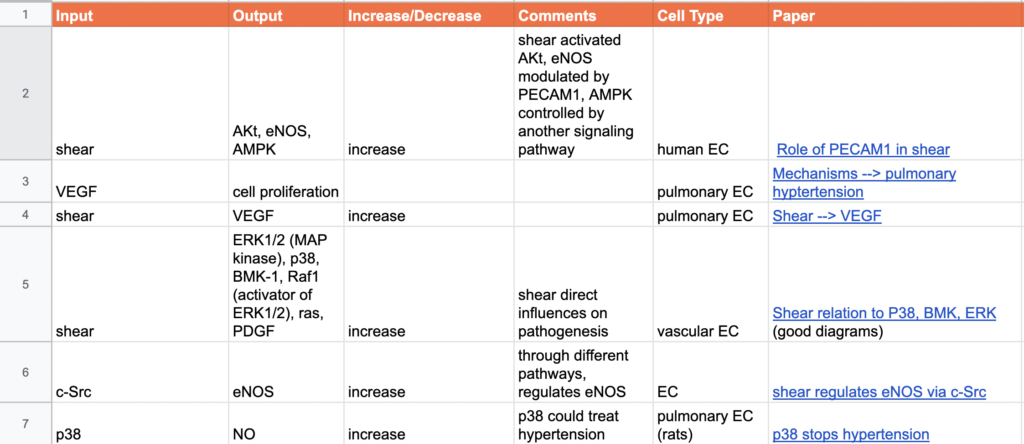
03/23/2022 - Getting Data
With Henrique and Melody, focused on collecting data from ArrayExpress that focused on hypoxia (shear stress was unavailable). Compared my boolean network to Henrique’s to determine potential new connections I needed to examine. Worked in terminal to analyze the data downloaded from ArrayExpress i.e. find transcription factors from genes. Confirmed connections of Alk5 –> Smad2/3 as well as P2 –> Calcium. Added to input/output table
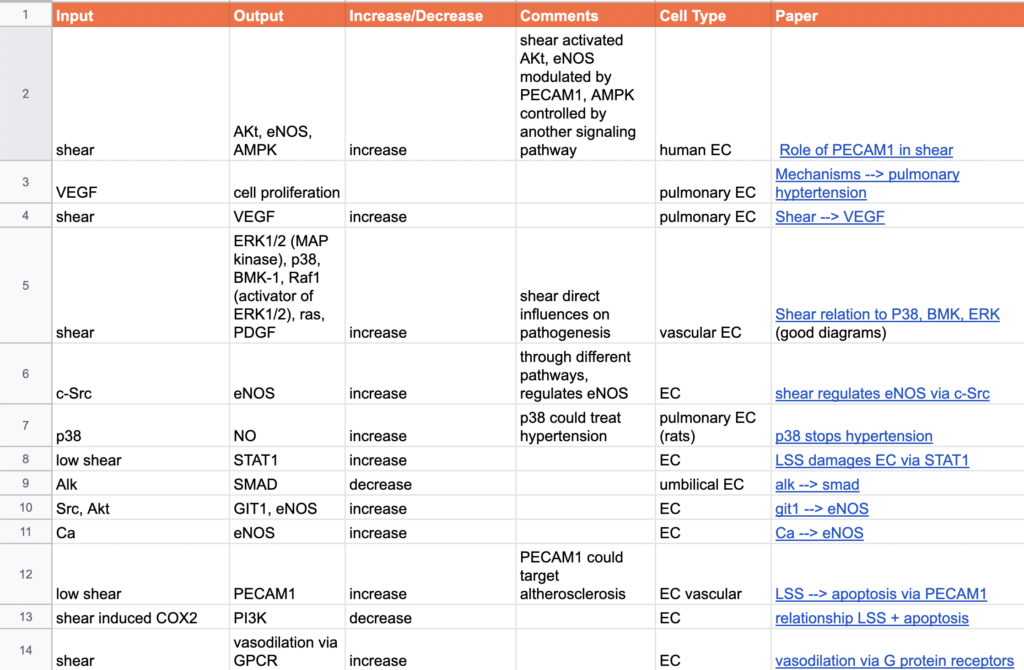
04/01/2022 - Looking at Databases
At start of meeting, discussed papers Sharanya and I found interesting. I talked about connections with p38, BMP, and ERK and the effects of the connections in model. For example, began to look at how low shear stress contributes to vasoconstriction, hyperplasia, and increased vascular permeability.
During meeting, Henrique created databases from the data collected last week. Used MySQL to analyze it further with Melody and Henrique. Used ChEA3 to analyze the genes (hypoxia and normoxia forms) from the analysis (query of genes transcribed with >= 50% increase). Findings showed transcription factors that were most influential for the genes by ranking them (often unclear to determine manually). This may not be the dataset used in our actual model, but it was extremely helpful to understand how to collect and analyze the data.
04/08/2022: Introduction Edits
Added to introduction drafted a few weeks ago. Prepared for presentation at UF concerning the work we have been doing. We reviewed the introductions. Edits I need to make are focusing on the impacts of low shear stress (other than hypertension or lung transplants). Below is the full introduction I presented…
“Pulmonary endothelial cells (ECs) line the interior of blood vessels and faciliate pulmonary circulation of nutrients and fluid transfers [1]. Endothelial cell vitality is directly affected by shear stress, a physical force or pressure against the cell walls, through the transduction of a series of intracellular signals (a signaling cascade), often noted as mechanotransduction. Shear stress plays a vital role in regulating pressure within the cell wall and has been seen to contribute to the ECs health [3]. A change in shear stress, due to the environment being in a hypoxic or ischemic state, has been seen to have effects on the endoethlium and consequently, the endothelial cells. This study aims to understand the chemical pathway that is affected by shear stress, in relation to endothelial cells, to ultimately create a mathematical model and network to represent the effects of manipulating shear stress related mechanisms on the endothelial cells.
Changes in shear stress initiate cascades that are received by the endothelium via mechanosomes and molecules such as PECAM and VEGFR2; this “message” results in NADPH activation, intracellular Ca2+, eNOS activation, and reactive oxygen species (ROS) like hydrogen peroxide production. When there is too much shear stress, in other words, too much production of ROS, there is a chance of the ECs becoming damaged and negative implications with cellular functions [6]. The excess ROS production can lead to autoimmune diseases, oxidative injury, or inflammatory cells. By that same token, the stop of shear stress, a hypoxic state in which there is no blood flow, often the case in transplants, leads to a reduction in nitric oxide, NO, and eNOS expression, both crucial to protecting and regulating the ECs.
Understanding shear-induced mechanotransduction is critical as this plays a major role in the function of arteries and is connected to growth factors, hyperpolzarizing factors, and hemodynamics [2]. According to Johns Hopkins Medicine, “around 40 percent of lung transplant recipients will experience an episode of acute rejection within the first year” [1]. The high rejection and infection rates could be attributed to the fact that the lungs have a stronger immune response compared to other organs [5]. Thus, understanding the change in shear stress for the application of this research has the potential to positively affect the vitality of lung transplants. Furthermore, shear stress also has effects on pulmonary hypertensios.
Better understanding the effects of shear stress on endothelial cells contributes to more accurate interpretations of the extracellular matrix as well as working towards finding more effective alternatives for approaching lung transplantation.”
Sources:
Johns Hopkins. “Long Term Complications.” Johns Hopkins University. https://www.hopkinsmedicine.org/transplant/programs/lung/_docs/4_Lung_Guide_longterm_complication.pdf.https://www.hopkinsmedicine.org/transplant/programs/lung/_docs/4_Lung_Guide_longterm_complications.pdf.
Chatterjee, Shampa, and Gary F. Niema et al. “Shear stress-related mechanosignaling with lung ischemia: lessons from basic research can inform lung transplantation.” Sep. 19, 2014. Am J Physiol Lung Cell Mol Physiol, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4217004/.
Yi-Shuan J Li et al. “Molecular basis of the effects of shear stress on vascular endothelial cells.” Oct. 2005, PubMed, https://pubmed.ncbi.nlm.nih.gov/16084198/#:~:text=Endothelial%20cells%20(ECs)%20are%20subjected,as%20well%20as%20gene%20expression.
Russo T.A. et al. “Altered shear stress on endothelial cells leads to remodeling of extracellular matrix and induction of angiogenesis.” Nov 19, 2020. Plos One. https://journals.plos.org/plsone/article?id=10.1371/journal.pone.0241040#:~:text=Endothelial%20cells%20(ECs)%20are%20subjected,morphology%2C%20physiology%20and%20gene%20expression.
Uzarski Joseph S.et al.“Adaptation of Endothelial Cells to Physiologically-Modeled, Variable Shear Stress.” Feb 14, 2014. PubMed. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3573044/
Other links that need to be cited properly:
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0241040#:~:text=Shear%20stress%20influences%20endothelial%20cell,molecules%20related%20to%20vascular%20biology.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3924805/#:~:text=Shear%20stress%20is%20%E2%80%9Csensed%E2%80%9D%20by,%2C%20vascular%20endothelial%20(VE)%E2%80%93
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5867026/
https://www.ahajournals.org/doi/10.1161/01.atv.0000125114.88079.96#:~:text=eNOS%20and%20Atherogenesis&text=A%20substantial%20body%20of%20evidence,acts%20as%20anti%2Datherogenic%20molecule.&text=NO%20from%20eNOS%20inhibits%20leukocyte,are%20important%20steps%20in%20atherogenesis.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851404/
04/15/2022 Creating a Presentation
Presented the start of the slides for presentation at UF (May 10). Melody informed us that we should present in one slide and should discuss the project rather than rely on words on the slides. Edited by slideshow to be condensed to one slide (shown in blue slide)
Also went over potential questions the panel may ask and the point of using a boolean network (our data is qualitative (on or off), so it is difficult to put a quantitative value on the relationship since it is a cascade). Went over our program in R and discussed the genes collected and what that represented (using SQR).
05/06/2022 - Rehearsing Presentations
We both presented our slide as a practice. We get 5 minutes to present on May 10th and my time was 4:59. I mainly took notes of the main points I wanted to cover (why we wanted to study low shear stress effects on endothelial cell signaling cascade, the purpose of using a boolean network, explaining a few of the connections in the network, and the future work of testing the model with genes).
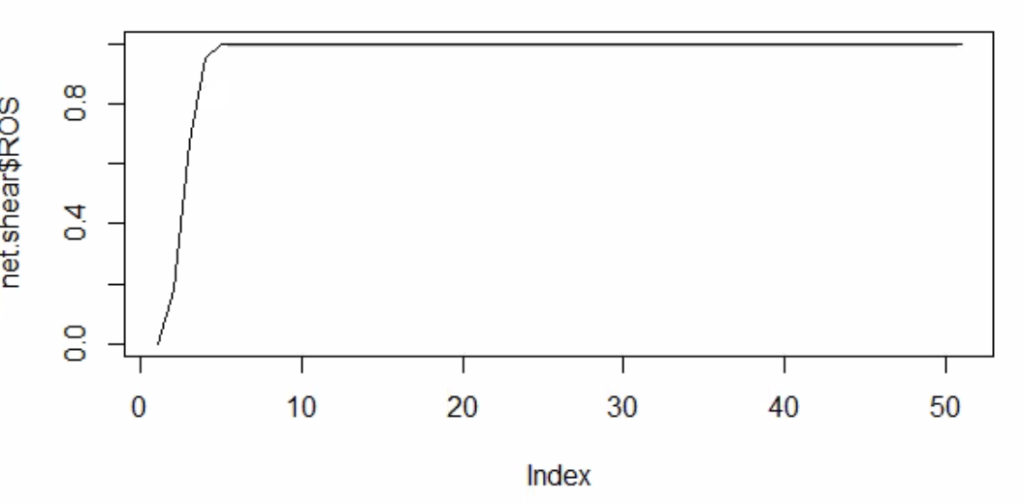
When Melody and Henrique asked questions, I was unsure on how exactly the boolean network worked. I now know that the simulation runs 1000 boolean networks with each iteration running 50 time steps. The results are then displayed on a chart with the average activation of a certain node or element compared to the activation over the 1000 runs.
05/13/2022 - Combining Models/Paper
cheduling over the summer
Discussed different types of research journals to publish in i.e. not predatory, top-tier = best (Science or Nature), or others on PubMed. Because I have been changing my model to fit the downregulation of shear stress, we decided to combine models since hypoxia and shear can be related in ECs.
Looked at different graph distributions in the network. Continuing to update model and network.
05/18/2022 - 06/17/2022 Combining Models/Paper
The past month, we have been combining models using Miro. We originally had trouble combining the models since only a few of our molecules were in common i.e. BMP4, P13K/AKT, eNOS. We also tried to consolidate our molecules so that it is easier to read. For instance, we had PI3k/Akt as seperate molecules, but we found they do similar paths, so we put them together in the model. Steps like these consolidated our work. I also continued to add to my input/output table to justify the connections. We found different connections that would tie in both shear and hypoxia. Melody and Henrique gave us helpful tips on how to find more molecules that were related: if they were related to hypoxia and eventually a molecule was activated from shear, they could create an “effect.” We developed different outcomes to answer the “so what” questions in our model (noted in the orange boxes at the bottom). So, every meeting for this month was used to discuss how to edit our new combined model and some research on literature that validated new connections.
Specifically, I am looking to see if Rak is connected since Ras and Raf are.
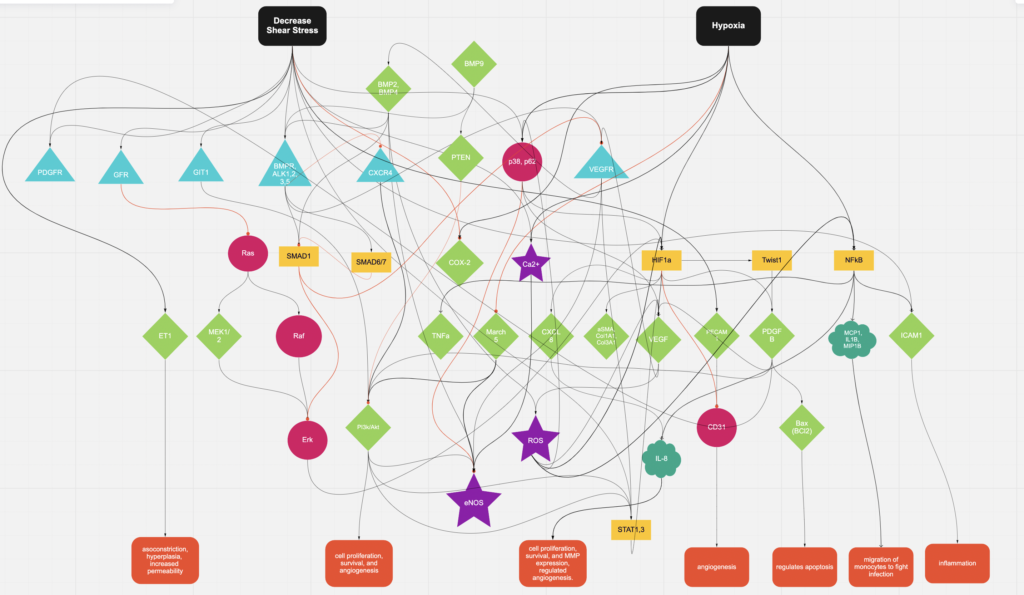
Some of the connections that related Hypoxia to Shear stress we found…
https://www.jbc.org/article/S0021-9258(19)34364-9/fulltext
- HIF1a → ET1
https://www.spandidos-publications.com/10.3892/mmr.2020.11060
- Shear stress → PECAM1
https://www.sciencedirect.com/science/article/pii/S0167488918300296
- Shear stress → eNOS
06/24/2022 - Combining Code
06/24/2022 - Combining Code
We combined our code so that it accurately represented the model. We also drafted a description of the network. Henrique gave comments on things to look into for the network/model.
–VEGFR leads to BMP2 (there is an intermediate we need to find)
–check if Ras is always on if it is not inhibited
–how does BMP9 and BMP1 have opposite purpose but same receptor (read more)
–does the network have attractors (in a boolean network, you stay in a cycle)

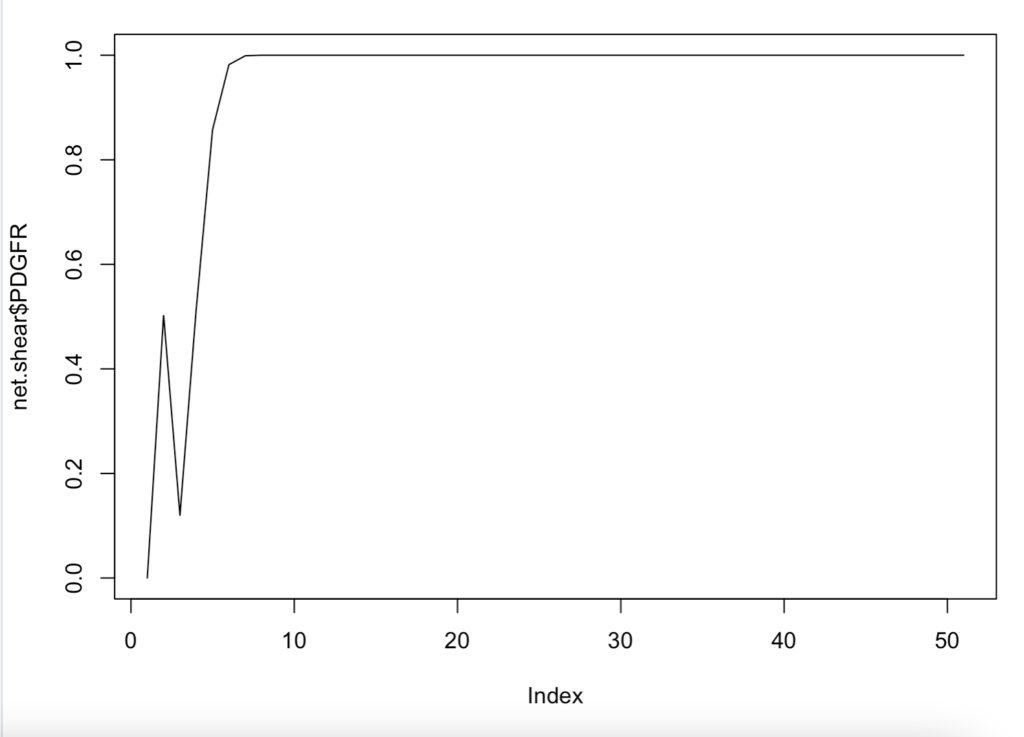
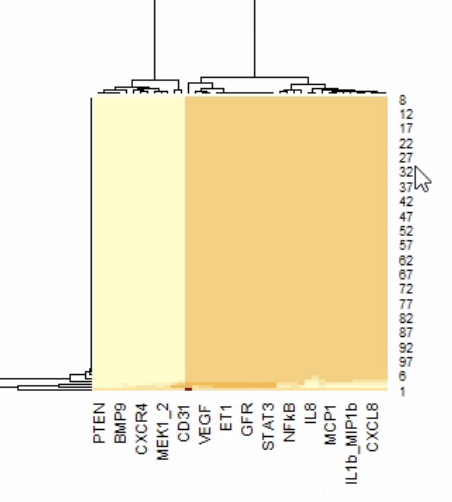
Above is an interesting representation of the relationship between shear and PDGFR. This is when we were trying to see which molecules were attractors.
07/01/2022 - Editing Code
07/01/2022 - Editing Code
Edited our code with Henrique to see which molecules were really attractors (we found most of the molecules to remain on, but had doubts if this was correct). Loaded interesting visualizations of the data.